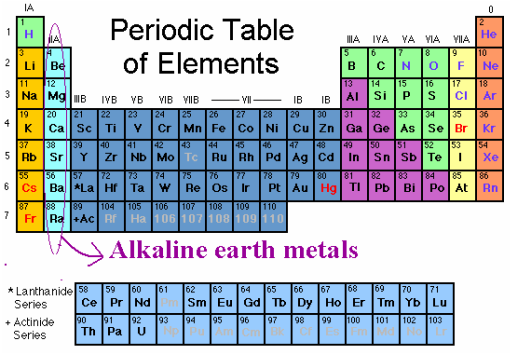
Alkaline Earth Metals

Prior to the 19th century substances that were nonmetallic insoluble in water and.
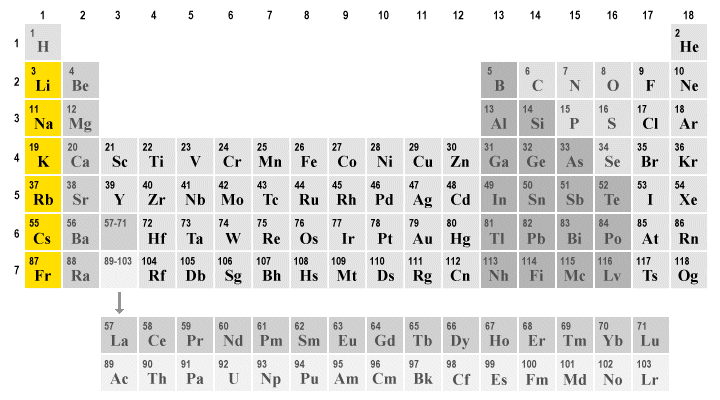
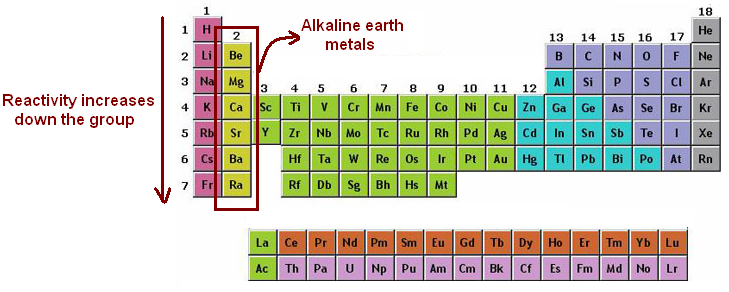
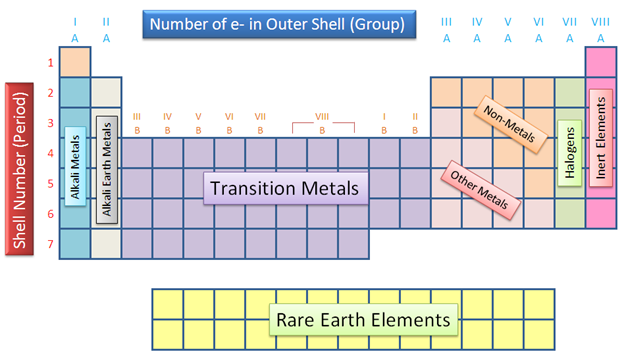
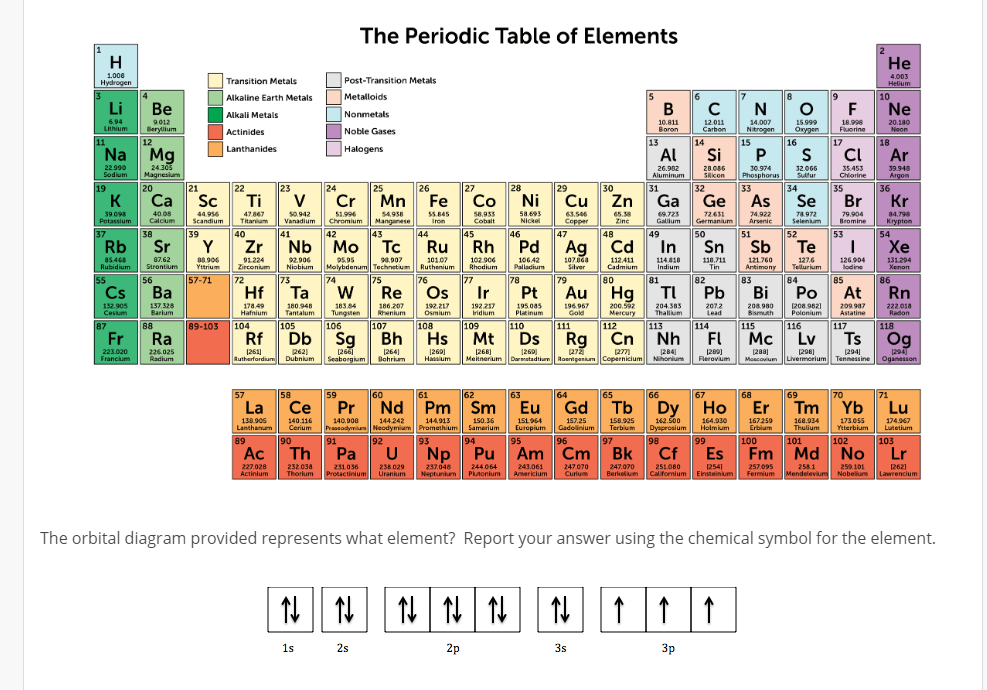
Alkaline earth metals. They are common in a wide variety of compounds and minerals. The alkaline earth metals are very reactive although less so than the alkali metals. The alkaline earth metals are six chemical elements in group 2 of the periodic table. Click the links or see below for more details on each.
Objective familiarize with the the periodic table solved label these groups of the difference between alkali metals and chem4kids elements periodic table alkaline earth metalsgroup 2 elements alkaline earth metals emedicalprep6 uses of alkaline earth metals in daily life pounds az chemistryalkaline earth metals the periodic tablealkaline earth metals definition location in the periodic. The elements of this group are quite similar in their physical and chemical properties. All the alkaline earth metals readily lose their two outermost electrons to form cations with a 2 charge. What are the similar properties of alkaline earth metals.
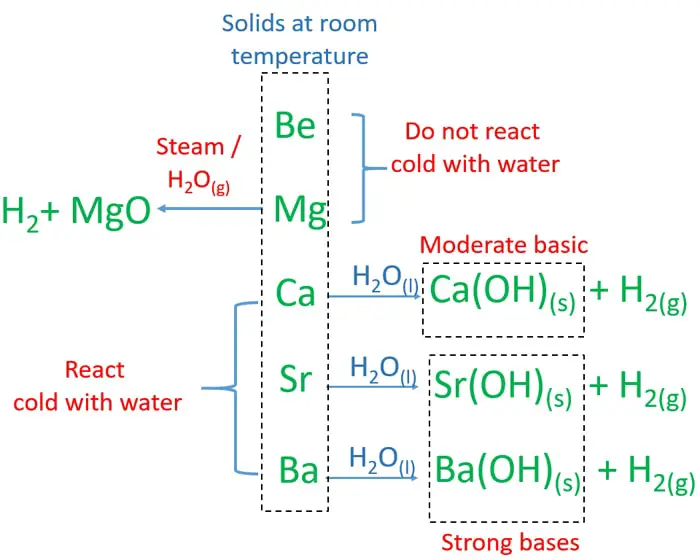
Alkaline earth metal any of the six chemical elements that comprise group 2 iia of the periodic table. All of the alkaline earth metals except magnesium and strontium have at least one naturally occurring radioisotope. Three novel alkaline earth metal salts mg2 1 ca2 2 sr2 3 of 5 5 dinitramino 3 3 methylene 1h 1 2 4 bistriazolate dnamt have been synthesized in a simple and straightforward manner. The alkaline earth metals are shiny silvery white and somewhat reactive metals at standard temperature and pressure.
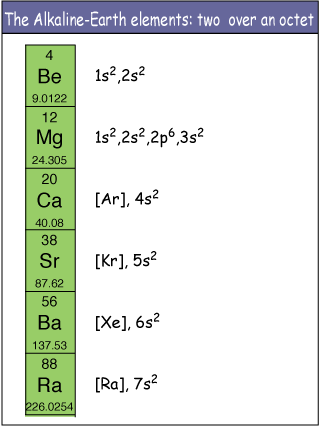
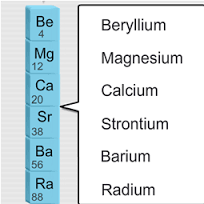
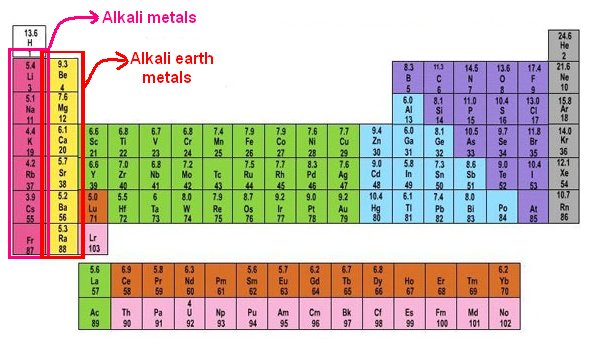
Ppt look at the following patterns alkaline earth metal alkali and alkaline earth metals alkali metal elements properties group 2 alkaline earth metals lab what are the properties of alkaline earth metalsalkaline earth metalsthe properties of alkaline earth metals group 2 a sciencealkaline earth metalsalkaline earth metals definition properties characteristicsalkaline earth metalswhat is the. Magnesium calcium strontium dan barium alkaline earth metal with sodium and potassium alkali metal identified in the first decade of nineteenth century by english chemist sir humphry davy on 1778 to 1829. Alkaline earth metals share many similar properties including. They are beryllium be magnesium mg calcium ca strontium sr barium ba and radium ra.
Because of their high reactivity the alkaline earths are not found free in nature. The elements have very similar properties. This group of elements includes beryllium magnesium calcium strontium barium and radium. The elements of the alkaline earth metals include beryllium magnesium calcium strontium barium and radium.
The crystal structures of 1 3 were confirmed by single crystal x ray diffraction revealing that the coordinated n. They are all shiny silvery white somewhat reactive metals at standard temperature and pressure. The elements are beryllium be magnesium mg calcium ca strontium sr barium ba and radium ra.

:max_bytes(150000):strip_icc()/alkalineearth-56a12cd75f9b58b7d0bcca7e.png)